CDV invests US$4 million in a new brucellosis vaccine plant in Argentina
New brucellosis vaccine plant in Argentina
CDV invests US$4 million in a new brucellosis vaccine plant in Argentina
CDV announced the opening of its new exclusive production plant for brucellosis vaccines, located at Plant 1 in the Pilar Industrial Park. With a total investment of US$4 million in civil works, plant services equipment, and production equipment, the building covers 300 m² and will achieve, in its initial production phase, a production capacity of 10 million doses of brucellosis vaccines.
This new exclusive production area for brucellosis vaccines is part of a package of major investments the company is making in Argentina.
Production and Availability
 The modern facilities at the brucellosis vaccine manufacturing plant comprise two completely independent production areas for the production of the two different types of Brucella, strain 19 and strain RB51: a quality control laboratory and a service area.
The modern facilities at the brucellosis vaccine manufacturing plant comprise two completely independent production areas for the production of the two different types of Brucella, strain 19 and strain RB51: a quality control laboratory and a service area.
Production began in July 2024, and the laboratory’s products are projected to be available starting in September 2024.
Market Share
“With this new production line, we estimate continued growth and reach a 35% market share. Of the total production, 70% will be destined for international trade, primarily to countries in Latin America, Asia, and the Middle East. “This is a huge step for Laboratorio CDV in our mission to expand into new markets and meet the demand for quality products, in pursuit of animal prevention and health,” stated Juan Roô, General Manager of Laboratorio CDV.
The firm’s executive stated that “the decision to move forward with this new plant responds to the need to increase production capacity due to the growth in orders for aquaculture vaccines—until now, they were produced alternately in the same plant as Brucellosis vaccines—the demand for Brucellosis Strain 19; and the obtaining of registrations abroad for Brucella RB51.” In Argentina, Senasa is leading a plan to control and eradicate brucellosis,” Roô concluded.
Expansion Project
CDV Laboratory is immersed in an ambitious expansion project into new international markets as a fundamental part of its business and growth strategy.
With the goal of expanding its reach and consolidating its global presence, CDV seeks to identify new opportunities, foster international collaborations, and make its products available with innovative and highly competitive technology in an international context, demonstrating its firm commitment to animal health prevention.
CDV Laboratory aims to be the first company in Latin America to obtain certification under the PIC/S (Pharmaceutical Inspection Co-operation Scheme) standards, a process it began in 2019.
The PIC/S (Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme) Standards are a set of international guidelines designed to harmonize inspection practices and ensure the quality of pharmaceutical products.
These standards are developed and maintained by the Pharmaceutical Inspection Convention and the Pharmaceutical Inspection Co-operation Scheme, an international organization that brings together drug regulatory authorities from various countries and regions.
To achieve this, in addition to its new brucellosis vaccine plant, the recently awarded Best Animal Health Company in Latin America 2023 (Animal Health Awards) has made an initial investment of US$60 million in a third veterinary vaccine manufacturing plant, designed to meet international World Class Manufacturing standards.
Another vaccine plant by 2025
This third veterinary vaccine manufacturing plant, expected to open in the first half of 2025, will not only meet these rigorous requirements but will also adopt a “green industry” approach that will optimize resources and improve production efficiency. The plant will integrate design, technology, and innovation in three key areas: construction, equipment, and efficient management of natural resources.
Meanwhile, the firm, present in Argentina for almost 40 years, continues to invest in facility improvements, new technologies, and research to continue providing excellent products in the veterinary sector, supplying the Argentine market, and becoming a recognized leader in international markets.
Brucellosis, a zoonotic disease of global threat
Brucellosis is a bacterial disease that primarily affects cattle and dogs. People contract it through contact with infected animals or contaminated products, especially unpasteurized milk.
According to the WHO, it is one of the most widespread zoonoses, with serious consequences for public health in endemic areas. The expansion of the animal industry and urbanization increase the risk. Although it is rarely transmitted between people, it poses an occupational hazard for livestock workers. Vaccination and serological testing are key to prevention. Pasteurizing milk, avoiding unpasteurized dairy products, and maintaining hygiene measures when handling animals and their products are also crucial.
The symptoms of brucellosis in humans are similar to those of the flu, including fever, weakness, malaise, and weight loss, although they may present atypically and vary in severity. The incubation period can vary from one week to two months, generally between two and four weeks. Identification and treatment of the disease can be complicated by the mild presentation of symptoms in some patients.
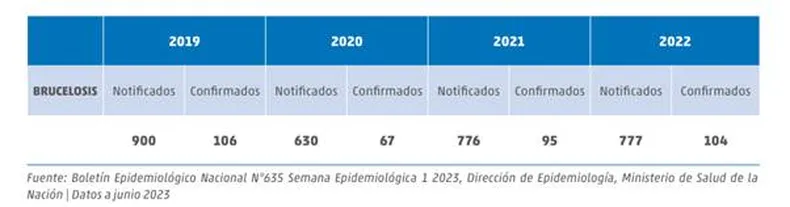
The National Health Surveillance System (SNVS) is currently used for the surveillance and reporting of human brucellosis cases. According to the latest data, 777 cases were reported in 2022, of which 104 were confirmed as brucellosis, as shown in the following table.
Health Plan
The current health plan requires annual immunization of all calves between 3 and 8 months of age with the brucellosis vaccine strain 19. This plan has successfully reduced the prevalence to 0.8% of animals, according to the latest data from SENASA.
In the region, several countries have reincorporated strain 19 into their health schedules. The latest example is Costa Rica, which last year purchased more than 100,000 doses of the CDVac Brucellosis vaccine strain 19, developed by Laboratorio CDV, to begin a new disease control plan in the country.
About Laboratorio CDV
CDV is a leading Argentine laboratory specializing in the development of biological products for the prevention of diseases affecting cattle and sheep herds in the country. Its line of prevention-oriented products and services is the most comprehensive on the market, comprising viral and bacterial vaccines, foot-and-mouth disease vaccine, reagents, and diagnostic services.
A pioneer in the development of a vaccine for the control of Bovine Viral Diarrhea using domestic strains and incorporating a double emulsion adjuvant to enhance immune response, it is also one of the first veterinary disease diagnostic centers and a member of the Senasa Laboratory Network. Since 2002, it has innovated in the production of biologicals for aquaculture and is the only laboratory with its own Diagnostic Laboratory. This has allowed it to understand the problems of the main diseases affecting livestock production and thus develop products tailored to the region’s specific circumstances.
In January 2020, the British magazine Animal Pharm recognized CDV as the “Best Company in the Veterinary Sector in Latin America in 2019.” CDV exports its products to more than 15 countries. In 2023, the laboratory was recognized for the second time as the Best Company in Latin America at the Animal Health Awards.
CDV has two modern vaccine production plants and a third under development, located in the Pilar Industrial Park, Buenos Aires Province:
Plant 1: Since 2003, it has produced vaccines for the prevention of reproductive, respiratory, clostridial, keratoconjunctivitis, and neonatal enteric complex diseases, as well as vaccines for important zoonotic diseases such as brucellosis, rabies, anthrax, and leptospirosis.
It also produces diagnostic reagents for the detection of tuberculosis and brucellosis, and vaccines to prevent diseases in birds and salmonids. The Diagnostic Laboratory, part of the Senasa network, is located on the same premises. It has a total annual production capacity of 150 million doses: 85 million doses for bovine vaccines and the remainder for salmonid and poultry vaccines.
Plant 2: The plant has been producing exclusively foot-and-mouth disease vaccines since 2018. This plant features state-of-the-art equipment and a high degree of system automation to ensure compliance with the most demanding global quality and biosafety standards. Current production capacity ranges between 2.5 and 5.0 million doses per month, depending on the vaccine format (tetravalent and bivalent, respectively).
Plant 3: Currently under construction and expected to be operational by the end of 2024, it will expand the production capacity of Plant 1 and will be able to produce products for animal health and disease prevention in new species: vaccines for pets, companion animals, and pigs.
The total projected production capacity, together with the production of Plant 1, will reach 280 million doses, of which 190 million are cattle vaccines (destined for the domestic market and export) and the remainder are vaccines for salmonids and other species such as pigs and companion animals. To learn about the progress of the CDV Laboratory’s Vaccine Plant 3 and follow its construction live, visit: https://cdv.com.ar/live/
SOURCE: Motivar